
The covalent radius of fluorine of about 71 picometers found in F 2 molecules is significantly larger than that in other compounds because of this weak bonding between the two fluorine atoms. The bond energy is significantly weaker than those of Cl 2 or Br 2 molecules and similar to the easily cleaved oxygen–oxygen bonds of peroxides or nitrogen–nitrogen bonds of hydrazines. The fluorine–fluorine bond of the difluorine molecule is relatively weak when compared to the bonds of heavier dihalogen molecules. In contrast, the diatomic molecules of the neighboring element oxygen, with two unpaired electrons per molecule, are paramagnetic (attracted to magnets). This makes it diamagnetic (slightly repelled by magnets) with the magnetic susceptibility of −1.2×10 −4 ( SI), which is close to theoretical predictions. While an individual fluorine atom has one unpaired electron, molecular fluorine (F 2) has all the electrons paired. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide and for still others (elements in certain groups) the highest oxidation states of oxides and fluorides are always equal.

įor many elements (but not all) the highest known oxidation state can be achieved in a fluoride. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases as well as a diverse set of organic compounds. Molecules containing fluorine may also exhibit hydrogen bonding (a weaker bridging link to certain nonmetals). Fluoride may act as a bridging ligand between two metals in some complex molecules.
Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Fluorine as straight (a) or bent (b) bridging ligands įluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1.
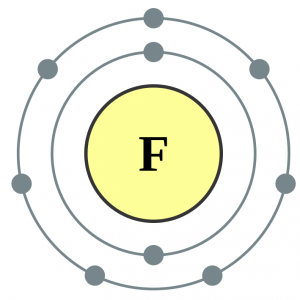
For applications and other aspects, see Fluorine. This article is about structural chemistry of the compounds of fluorine.


 0 kommentar(er)
0 kommentar(er)
